Tablet Presentation
Each tablets contains:1. Each uncoated dispersible tablet contains:
Levocetirizine HCI IP 2.5mg
Montelukast Sodium IP Eq. to
Montelukast 5 mg
2. Each film coated tablets contains:
Levocetirizine HCI IP 2.5 mg
Montelukast Sodium IP Eq. to
Montelukast 4 mg
Montelukast is a Leukotriene receptor antagonists.(is used for maintenance treatment of asthma and to relive symptoms of seasonal allergies) It is a selective and orally active leukotrien antagonist that inhibits the cysteinyl leukotrien CysLT1 receptor.
Levocetirizine is a Third — generation non — sedative antihistamine.(is prevents the release of other allergy chemicals and increased blood supply to the area, and provides relief from the typical symptoms of hay fever). Levocetirizine, the R — entaiomer of Cetirizine, is a potent and selective antagonist of peripheral H1 — receptors.
Indications
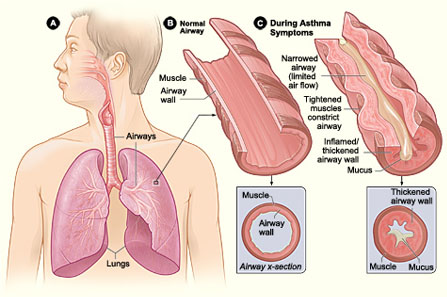
- Proplylactic treatment of asthma (Asthma is a disorder that causes the airways of the lungs to swell and narrow, leading to wheezing, shortness of breath, chest tightness and coughing).
- Chronic treatment of asthma associated with allergy.
- Seasonal and perennial rhinitis.
Drug Interactions
BEFORE TAKING THIS MEDICINE TELL THE DOCTOR ABOUT ANY MEDICAL PROBLEMS AN ALLERGIES THAT CHILD HAS NOW OR HAS HAD.Some medicines may affect how patient's works, or other medicine work. If anyone is taking or has recently taken other medicines, including those obtained without a prescription.
Such as:
Phenobarbital: Used for treatment of epilepsy
Pheytoin: Used for treatment of epilepsy
Rifampicin: Used for treatment of Tuberculosis and some other interactions.
THEOPHYLLINE, PREDISOLONE, ORAL CONTRACEPTIVES, TERFENADINE, DIGOXIN, WARAFIN, THYROID HORMONES & SEDATIVE HYPNOTICS.
Side Effects
Thirst was the only adverse experience commonly reported as drug related in 2 — 5 yr olds. Cough was reported as frequently (>1/10) occurring in trials regardless of causalty.In older (6 — 14 yr), headache was the most commonly reported drug — related adverse — effect. The most commonly reported adverse reactions were headache, asthma and upper respiratory tract infections; however none were significantly different in frequency between the groups.ContraIndications
Hypersensitivity, pregnant patients, patients with blood and lymphatic system disorder and psychiatric disorder.Conclusion
From the above discussion, it can be concluded that this medicine could improve the quality of life of children with allergic rhinitis as well as asthma in addition to being efficacious as immunotherapy in seasonal allergic rhinitis in children aged 2-5 years. Moreover it has a considerable safety profile, with persistent results. Oral doses of this medicine has shown efficacy as a preventive treatment for asthma during clinical trials in children aged 2 — 14 years. This combination offers as effective, well tolerated and convient treatment option for children with asthma and allergies.Know About new DCGI Approved Products
Copyright © by Alaina Pharma. All rights reserved.
Created by Alaina Pharma